Understanding Treatment Costs & Outcomes in Parkinson’s Disease
This large-scale Medicare-based study, conducted by ACT founder Dr. Renée Arnold and colleagues, reveals key insights about how different formulations of carbidopa–levodopa—commonly prescribed for Parkinson’s disease (PD)—affect real-world healthcare costs, treatment patterns, and patient outcomes. This is an example of a retrospective burden of illness study based off of real data to demonstrate unmet need and value proposition.
This study explored:
- Patterns of use for four levodopa-containing regimens (IR, immediate release carbidopa/levodopa; IR+CR, immediate release + controlled-release carbidopa/levodopa; CR, controlled release carbidopa/levodopa; ER, extended release carbidopa/levodopa).
- How treatment persistence, switching, and dosage changed over time.
- The economic burden associated with each regimen and with disease severity.
- The use of additional medications (e.g., dopamine agonists, MAO-B inhibitors).
Levodopa Equivalent Daily Dose (LEDD) was used as a proxy for the severity of a patient’s Parkinson’s Disease. The study found that patients with higher LEDD had slightly longer treatment duration and better persistence; greater use of adjunct medications; and higher outpatient and prescription drug costs.
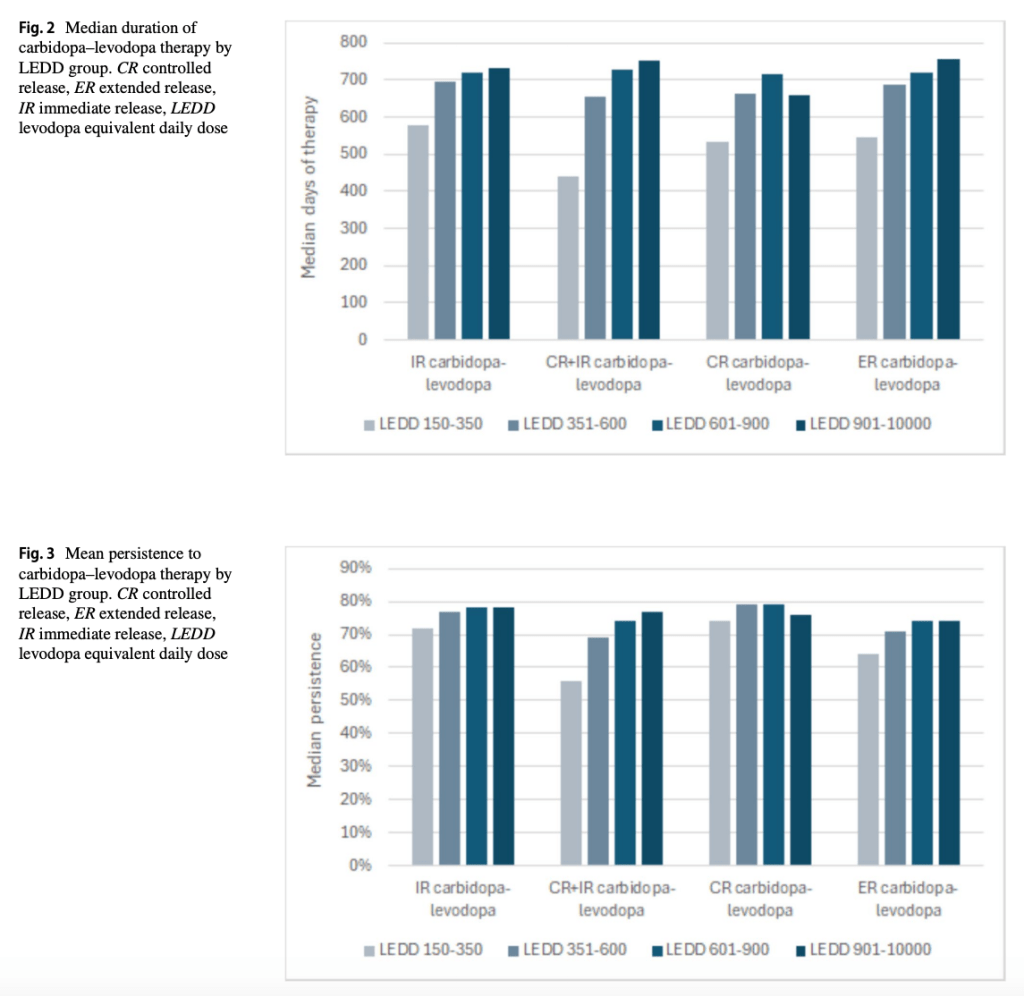
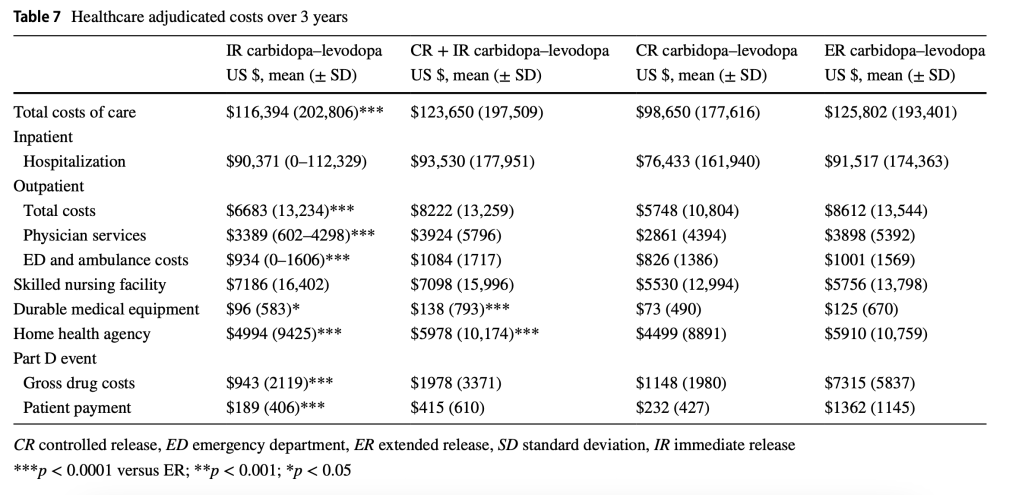
Total 3-year healthcare costs were lower for patients prescribed CR carbidopa–levodopa (US $98,650) compared with other formulations. Duration, persistence, and overall costs tended to increase with levodopa load, primarily driven by outpatient care and medication costs.
To read the full study, click here.
